- Values/interpretations for which delays in reporting can result in serious adverse outcomes for patients
- The scope includes:
- laboratory, cardiology, radiology, and other diagnostic tests
- in inpatient, emergency, and ambulatory settings.
Concept Schematic for Laboratory
(click on individual parts of map to hyperjump to relevant text section)
Communication of critical laboratory results is of vital importance in patient safety. It is a hospital-wide project encompassing:
- quality control by the clinical laboratory
- communicating critical test results to the appropriate clinical personnel
- recognition of abnormal test results by the patient care team
- appropriate action by the patient care team
- auditing to assess the appropriateness of clinical action
- creation and implementation of guidelines to prevent/mitigate diagnostic errors and inappropriate responses
- follow-up and continued care by the patient care manager/team to prevent recurrences and to ensure a smooth path to hospital discharge
This is not a project limited to the laboratory, and needs a leader capable of managing the interactions between laboratory staff, clinical physicians, and educational committees involved in the implementation of evidence-based medicine.
Safe Practice Recommendations
Safe Practice Recommendations [1]
- Identify who should receive the results
- The primary responsibility for receiving and following up on test results lies with the ordering physician
- Laboratory results must be reported directly to the physician who can take action, not to an intermediary.
- Identify who should receive the results when the ordering provider is not available
- Develop a procedure to link each patient with either a physician or a service at the time of admission
- Develop a call schedule/system that works to identify whom the results should go to when the ordering physician is not available
-
Role-based
coverage models have proven more reliable than traditional call systems.
→ link each patient with a position/service designated at admission and then have an on-call system tied to that position [1]
- Define what test results require timely and reliable communication
- Maintain a prioritized list of critical test values/interpretations that require accelerated notification systems
- Ensure the list is segmented into categories that correspond to differentiated notification time requirements
- Ensure the list is reviewed and verified at least annually and includes a process for adding/dropping tests
- Reference existing standards and evidence on criticality
- Limit the number of tests categorized as highest priority (red)
- Identify when test results should be actively reported to the ordering provider and establish explicit time frames for this process
- Define appropriate notification time parameters for communicating critical test results according to urgency, e.g. within 1 hour, within the shift (target 6-8 hours), within 3 days. Example (all categories require acknowledgement):
Red
category — requires stat pager, immediate clinical decision requiredOrange
category — results should be called; clinical decision required within hours.
• maximize efficiencies of workflow issues
• avoid unnecessary calls late at night
• synchronize calls with other existing systems e.g. change of shifts etc.Yellow
category — results can be sent passively; clinical decision required within days
- Describe explicit steps in notification system; describe when laboratories should initiate and follow up on notifying the ordering physician about critical test results
- Develop a fail-safe plan for communicating critical test results when the ordering physician cannot be contacted within the designated time frame.
- Hospitals should have fail-safe plans in place for reporting critical findings and to ensure that the patient will receive timely clinical attention!
- Define appropriate notification time parameters for communicating critical test results according to urgency, e.g. within 1 hour, within the shift (target 6-8 hours), within 3 days. Example (all categories require acknowledgement):
- Identify how to notify the responsible provider(s)
- Identify and utilize the communication techniques that are most appropriate for the particular clinical situation, e.g. active
push
system for results requiring a prompt clinical responseRed
category results should be called to the responsible physician; the physician must respond to a page- Results should not be left with secretary or nurse or just as emails
- Ensure acknowledgement of receipt of test results by a physician who can take action for all categories (red, orange, yellow) of critical test values/interpretations
- Systems should reliably ensure the hand-off to the responsible physician is complete if change of shift occurs
- Identify and utilize the communication techniques that are most appropriate for the particular clinical situation, e.g. active
- Establish a shared policy for uniform communication of all types of test results (laboratory, cardiology, radiology, and other diagnostic tests) to all recipients
- Make the notification system explicitly clear to all stakeholders
- Use
read-back
techniques in the process of acknowledging recipt of results - Develop a shared policy with the key elements of the quality improvement monitoring plan
- Plan for annual review and validation
- Use
- Encourage and foster shared accountability and teamwork across and between clinical disciplines
- Implement face-to-face interdisciplinary change-of-shift debriefings for the handoff of laboratory, cardiology, radiology, and other relevant clinical information e.g. problem lists, allergies, medications, a
to do
list - Describe relative responsibilities of the laboratory, cardiology, radiology, the ordering physician, the on-duty physician, and the nurse
- Address the importance of shared responsibility and partnering when facing a
red
category finding
- Implement face-to-face interdisciplinary change-of-shift debriefings for the handoff of laboratory, cardiology, radiology, and other relevant clinical information e.g. problem lists, allergies, medications, a
- Decide what information should be included as a minimal dataset to be communicated to the responsible person. Examples of a minimal dataset should include:
- This is a
red
(orange) category finding - Significant comorbidities
- Prior test results, if available
- Related medications
- Other relevant clinical information
- This is a
- Make the notification system explicitly clear to all stakeholders
- Design reliability into the system
- When ordering a test, include a minimal dataset of clinical information to support the interpretation of diagnostic tests
- Create tracking systems to assure timely and reliable communication of test results. Develop special procedures for situations where delays typically occur:
- After discharge
- Ambulatory (cross departmental boundaries)
- Late arriving
- Other predictable relevant situations (shift changes, after-hours, surgeon in OR, etc.)
- The responsibility for tracking and follow-up on positive findings lies with the relevant physician
- Explore the possibility that laboratory, cardiology, and radiology would monitor the receipt (acknowledgement) and document handoff of findings
- Support and maintain systems
- Partner with patients in the communication about test results.
Nothing about me, without me
- Include family as appropriate, given consideration to confidentiality and regulatory compliance
- Provide orientation and ongoing education on procedures for communicating critical test results to all members of the medical team
- Provide ongoing monitoring of the effectiveness of systems, e.g. weekly failure rates, tests of call systems, response times
- Partner with patients in the communication about test results.
- Support infrastructure development
- Adopt advanced communication technologies (intranet access, LINE messaging, email to patients with attention to confidentiality issues)
- Improve laboratory and other test system capabilities. Plan for integrated medical record solutions to link clinical information with laboratory results, as in the following:
- Drug-drug interactions
- Previous test results
- Enable reporting of complex threshold criteria such as renal and pediatric dosing
- Track trends in patient conditions
- Link to medical record to identify first diagnosis of cancer or diabetes
- Link documentation of acknowledgement fields to tracking reports to monitor feedback loop
- Evaluate the use of Point-of-Care (POC) testing in critical and ambulatory areas; integrate POC results with other test results and make them available to other physicians
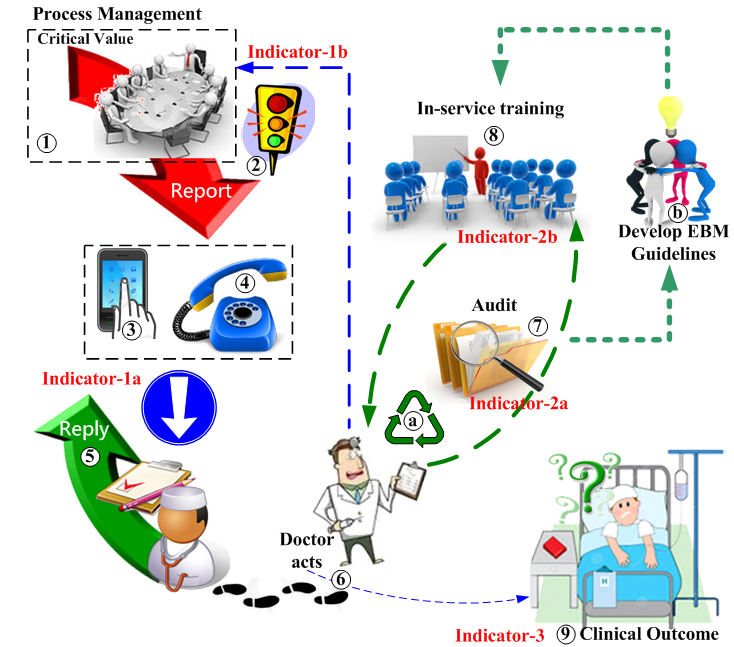
Manage the critical values database
① Manage the critical values database
- responsible for data validation/accuracy of laboratory results
possibly supported by laboratory accreditation such as: CAP ± CLIA.CAP: College of American Pathologists
CLIA: Clinical Laboratory Improvement Act - design, implement, and manage a hospital database of critical laboratory values:
- Although hospitals have thresholds about which results to notify, these nearly always include many results that are not truly critical, thus greatly increasing the number of calls and diluting the sense of urgency with which the notifications are received by physicians!
- start with hospital-wide, reviewed annually
- progress to specialty-specific
- compile, and store safely, evidence that the process is operational:
- minutes of meetings discussing management of critical values database
- up-to-date list of critical values
- documentation of requests to changes to critical values
Inform the medical team
② Inform the medical team of items that exceed the specified limit
- responsible for data validation/accuracy of laboratory results
possibly supported by laboratory accreditation such as: CAP ± CLIA.CAP: College of American Pathologists
CLIA: Clinical Laboratory Improvement Act - design, implement, and manage a hospital database of critical laboratory values:
- start with hospital-wide, reviewed annually
- progress to specialty-specific
- compile, and store safely, evidence that the process is operational:
- minutes of meetings discussing management of critical values database
- up-to-date list of critical values
- documentation of requests to changes to critical values
Confirm the medical team received and acted
⑤ Confirm the medical team received and acted
Audit compliance with standard guidelines
⑦ Audit compliance with standard guidelines
Develop EBM guidelines and in-service training
⑧ Develop EBM guidelines and train medical teams to use them
Clinical outcome and process indicators
⑨ Indicators for evaluating appropriateness and effectiveness
Project Monitoring Indicators
-
Notification success rate. Documented feedback from doctor that he was notified by the laboratory
- Physician directly acknowledges being notified by the laboratory
- Physician activates the order system in the digital medical record and confirms sighting of the laboratory warning
-
Appropriate management rate. Physician compliance with standard medical guidelines
- Audit results by laboratory item
- The number of new hosital guidlines created and implemented
-
Clinical (survival) rate. Patient survival by laboratory item
- at 48-hour after the laboratory made the first notification
- at the time the patient was discharged from the hospital
Tracking critical values
HIPAA Compliance [2]
What is HIPAA Compliance? HIPAA, the Health Insurance Portability and Accountability Act, sets the standard for protecting sensitive patient data. Any company that deals with protected health information (PHI) must ensure that all the required physical, network, and process security measures are in place and followed.
The HIPAA Privacy Rule addresses the saving, accessing and sharing of medical and personal information of any individual, while the HIPAA Security Rule more specifically outlines national security standards to protect health data created, received, maintained or transmitted electronically, also known as electronic protected health information (ePHI).
If you are hosting your data with a HIPAA compliant hosting provider, they must have certain administrative, physical and technical safeguards in place, according to the U.S. Department of Health and Human Services. The physical and technical safeguards are most relevant to services provided by your HIPAA compliant host as listed below, with detail on what constitutes a HIPAA compliant data center.
- Physical safeguards include limited facility access and control, with authorized access in place. All covered entities, or companies that must be HIPAA compliant, must have policies about use and access to workstations and electronic media. This includes transferring, removing, disposing and re-using electronic media and electronic protected health information (ePHI).
- Technical safeguards require access control to allow only the authorized to access electronic protected health data. Access control includes using unique user IDs, an emergency access procedure, automatic log off and encryption and decryption.
- Audit reports, or tracking logs, must be implemented to keep records of activity on hardware and software. This is especially useful to pinpoint the source or cause of any security violations.
- Technical policies should also cover integrity controls, or measures put in place to confirm that ePHI hasn’t been altered or destroyed. IT disaster recovery and offsite backup are key to ensure that any electronic media errors or failures can be quickly remedied and patient health information can be recovered accurately and intact.
- Network, or transmission, security is the last technical safeguard required of HIPAA compliant hosts to protect against unauthorized public access of ePHI. This concerns all methods of transmitting data, whether it be email, Internet, or even over a private network, such as a private cloud.
References:
- Hanna D, Griswold P, Leape LL, Bates DW. Communicating critical laboratory results: safe practice recommendations. Jt Comm J Qual Patient Saf 2005; 31(2): 68~80.
- Owen What is...HIPAA Compliance? www.otava.com 2019-11-4
- Thomas Pornin in answer to Paul Podlipensky How hard is it to intercept SMS (two-factor authentication)? security.stackexchange.com/questions/ 2012-2-8.